Brief Communication
Comparison of Foldscope to optical microscope to identify basic histology
Mike Yoshio Hamasaki1
1PhD, Faculdade Sírio-Libanês, Instituto de Ensino e Pesquisa and Universidade São Judas Tadeu, São Paulo, Brazil
ABSTRACT
Introduction: The teaching of basic histology is crucial in the training of healthcare professionals. Traditionally, the optical microscope is used, but it has limitations such as cost and the need for specific infrastructure. The Foldscope (FS), a low-cost and easy-to-handle tool, emerges as a promising alternative. Objective: To validate the efficacy of the FS in teaching basic histology for healthcare training, comparing it with the traditional method of binocular optical microscopy. Method: We used a kit of prepared histology slides, first examined with an optical microscope and then with the FS. We used a smartphone for image capture, ensuring comparative accuracy between the methods. Results: The FS could observe structures seen with a 10x objective in conventional microscopy but with less sharpness and some peripheral distortions. The applicability of the FS proved effective in observing structures of various tissues, such as the thyroid, arteries, veins, and neurons, although it has limitations for teaching muscular and testicular tissues. Conclusion: The FS is an innovative educational tool that bridges the gap between theory and practice in histology teaching. It is a complementary resource to the optical microscope, effectively identifying structures with about 100x magnification. However, it does not completely replace the optical microscope due to its limitations in magnification and image quality.
Keywords: Foldscope; Microscopy; Histology; Teaching Materials; Teaching Method
Date submitted: 26-Feb-2024
Email: Mike Yoshio Hamasaki (mike.yoha@gmail.com)
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Citation: Yoshio Hamasaki M. Comparison of Foldscope to optical microscope to identify basic histology. Educ Health 2024;37:152-157
Online access: www.educationforhealthjournal.org
DOI: 10.62694/efh.2024.32
Published by The Network: Towards Unity for Health
Introduction
Basic histology training is a fundamental step in the education of any healthcare professional. This curricular component aims to equip students with the ability to recognize and understand the histological structures of organs, with the goal that this knowledge assists in clinical practices in each professional area. The teaching of histology, along with other basic biomedical sciences, represents the foundation for a more advanced understanding of the human body and its pathological manifestations.
The optical microscope is the instrument traditionally used for teaching histology. However, this tool has several limitations, such as its high cost, the need for adequate lighting, a specific laboratory, and skilled labor to prepare classes, (typically laboratory technicians). The Foldscope (FS) is a new technology with the potential to overcome some of these limitations. It is a low-cost microscope made from paper folding and can be an interesting alternative to minimize the dichotomy that exists between practical and theoretical classes in the teaching of animal histology.
The FS, lightweight at only 8.8 grams and compact with dimensions of 70x20x2 mm, can be assembled swiftly in under a minute. It features adjustments along the X, Y, and Z axes, making it exceptionally suitable for field studies. The kit includes essential accessories such as paper slides and plastic coverslips. An LED with a diffuser or condenser lens provides illumination, powered by a watch battery, and can be attached to both the microscope and the smartphone, eliminating the need for external light sources in low-resolution imaging systems (Figure 1).
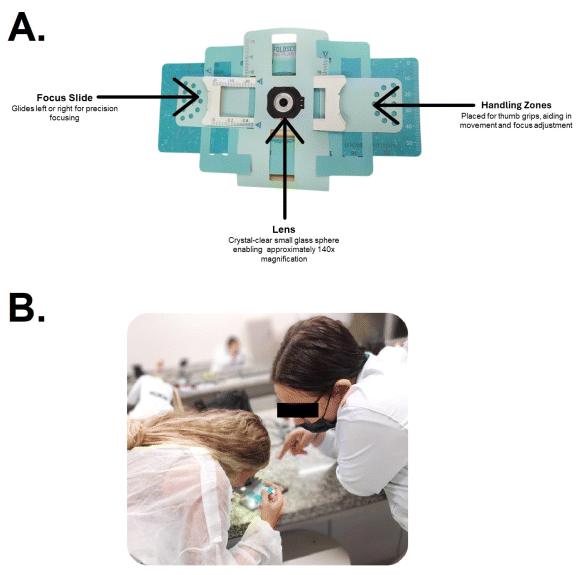
Figure 1 A: Foldscope Components – An Illustrative Overview of the Portable Paper Microscope. This figure depicts the unassembled Foldscope, highlighting its key parts: the lens with 140x magnification capability, focus ramp for adjusting clarity, water-resistant paper construction suitable for repeated cleaning, and thumb grip holes for ease of handling. B: Students Engaging with the Foldscope in a Histology Laboratory. The image captures an educational moment where students are using the Foldscope, a portable paper microscope, to examine histological samples.
The FS tool became available for use in 2014, and since then some studies have been conducted testing the efficacy of the FS in the field of medicine. These studies assess the applicability of the FS as a diagnostic tool for a variety of pathological conditions, including parasitic infections,1,2 fungal keratitis,3 pre-cancerous alterations4,5 and pneumonia associated with mechanical ventilation.6
In terms of comparing different microscopy methods, there are no studies to date that compare use of the FS to other microscopy methods to teach basic histology to students in the health area. Therefore, this study analyzes a variety of tissues, fundamental in the training of health professionals, to determine the efficacy and practicability of the FS compared to the traditional method using binocular optical microscopy.
Methods
To conduct the comparative tissue study, we used a prepared slide kit, model TIL-H-80, marketed by Opton Anatomic® (Sao Paulo, Brazil). This kit contains various slides already prepared for histological analysis. For this study, we selected some of these specific slides, including samples of the thyroid gland, testicle, artery, vein, tendon, neurons, trachea, and sections of cardiac muscle, skeletal muscle, and smooth muscle. The selection of these slides was based on representing at least one example of each fundamental tissue of the human body, often addressed as objects of study in histology classes.
In this work, we used the first version of the FS which provides approximately 140x magnification (equivalent to a 14x objective in a conventional microscope), with a resolution of 2μm and a field of view radius of 518μm. The methodology involved preliminary observation of the selected tissues using a binocular biological microscope, model 1600X BIO-100® (Biocentrix, São Paulo, Brazil), and subsequently, the same slides were analyzed using the Foldscope (Foldscope Instruments, California, USA). To make the comparison similar to the magnification capability of the FS, observations in the optical microscope were carried out under a 10x achromatic objective.
For the comparative tissue study, to obtain images from both optical microscopy and the FS, an iPhone 14 Plus smartphone (Apple Inc., California, USA) equipped with a 12MP wide-angle camera was used. This camera has a resolution of 4000 x 3000 pixels, optical image stabilization, 26 mm, f/1.5 aperture, sensor-shift optical image stabilization, a seven-element lens, and 100% Focus Pixels.
During the image capture, the same regions of the analyzed slides were photographed using both microscopic methods; this care was fundamental to equalize the obtained images, thus ensuring a precise and reliable comparison of the examined histological tissues. In the conduct of this study, the slides were analyzed by two members of the core of human body biosystems (basic health science educators) at our institution. Both possess over 10 years of experience in teaching histology. The decision to involve two professionals was specifically made to avoid biases in interpreting the results, which in this study only underscore aspects of the agreement between the two analyses. It is important to note that the analyses of the slides were not conducted blindly, allowing the reviewers to leverage their expertise to recognize inherent differences between microscopic methods.
The study was conducted during the second semester of 2023 at Universidade São Judas Tadeu, specifically at the campus located in São Bernardo do Campo, in the city of São Paulo, Brazil. This location provided a suitable setting for conducting the histological analyses and comparing the microscopic methods discussed in the research.
Results
Our findings indicate that structures visible under a 10x objective in conventional microscopy can also be observed using the FS. However, the FS shows reduced sharpness compared to traditional microscopy. Images generated by the FS typically exhibit good sharpness at the center but significant distortions at the periphery, likely due to the spherical lens used.
In the analysis of thyroid slides, it was possible to identify the capsules, thyroid follicles, and colloids (Figure 2A). In testicular tissue, only the seminiferous tubules were visible (Figure 2B). Important structures for education, such as Sertoli cells, spermatogonia, primary and secondary spermatocytes, and spermatids, were not observed.
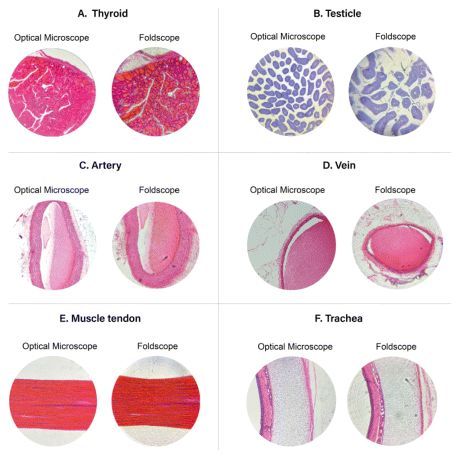
Figure 2 A: Thyroid gland stained with hematoxylin and eosin, viewed under 10x optical microscope and 140x Foldscope magnification. B: Testicle in toluidine blue, viewed under 10x optical microscope and 140x Foldscope magnification. C: Cross-sectional view of an artery in hematoxylin and eosin, viewed under 10x optical microscope and 140x Foldscope magnification. D: Cross-sectional view of a vein in hematoxylin and eosin, viewed under 10x optical microscope and 140x Foldscope magnification. E: Longitudinal section of a tendon in hematoxylin and eosin, viewed under 10x optical microscope and 140x Foldscope magnification. F: Cross-sectional view of the trachea in hematoxylin and eosin, viewed under 10x optical microscope and 140x Foldscope magnification.
In histological sections of arteries and veins (Figure 2C and 2D),, structures were revealed by both microscopic techniques. In the FS, the tunica media and adventitia were discernible in the artery with 14x magnification. In veins, the three tunics (intima, media, and adventitia) were visible, although there was no consensus among the reviewers regarding the clarity in delineating the tunica intima.
In the longitudinal section of the tendon, Figure 2E, the FS revealed the parallel organization of protein fibers in the extracellular matrix of this connective tissue, as well as the hematoxylin staining of the nuclei of probable fibroblasts. However, the peripheral distortion of the images was quite evident.
Analysis of the trachea (Figure 2F) with the FS highlighted essential elements to understand its morphological characteristics, including the lining epithelium, connective tissue, mucous gland, hyaline cartilaginous tissue, and muscle fibers. Microscopy of the trachea using the FS proved to be an educationally important avenue for an understanding and the practical application of the knowledge of various basic tissues.
In the study of neurons (Figure 3A), the FS provided a clear visualization of the cell body, dendrites, axons, and nuclei of glial cells. This detailed observation assisted in the analysis of the complex histological organization of neurons.

Figure 3 A: Motor neurons stained with toluidine blue, viewed under 10x optical microscope and 140x Foldscope magnification. B: Longitudinal section of cardiac muscle in toluidine blue, viewed under 10x optical microscope and 140x Foldscope magnification. C: Longitudinal section of skeletal muscle in toluidine blue, viewed under 10x optical microscope and 140x Foldscope magnification. D: Longitudinal section of smooth muscle stained with hematoxylin and eosin, viewed under 10x optical microscope and 140x Foldscope magnification.
Finally, the analysis of striated muscle tissues (Figure 3B and 3C) allowed the visualization of the cylindrical and elongated shape of muscle cells, being branched in cardiac tissue and unbranched in skeletal. However, features such as striations in the cytoplasm were not observed. In smooth muscle (Figure 3D), it was not possible to discern the spindle-shaped fibers, the central and unique nucleus, and the absence of striations in the cytoplasm with the FS. Therefore, the functionality of the FS for teaching muscle tissues appears limited, and its use is not recommended for such purposes.
DISCUSSION
The study most comparable to our work was conducted by Hernández-Pérez and Nieto-Sobrino in 2022.7 They examined the opinions of 92 Plant Histology students at the University of Salamanca on the use of the FS. Student responses indicated that the FS was efficient in observing cellular structures and was well-received, being considered an appropriate tool for undergraduate settings, with recommendations for its implementation in future classes.
While there are similarities, the previously mentioned study focused on the use of the FS in higher education to examine plant histological structures, such as onion cells, tilia sp. and cannabis sativa. The FS showed efficacy in identifying certain aspects but did not include a detailed comparison or validation against traditional optical microscopy. Although the educational applications are similar, the scopes and methodologies of the two studies differ.
In the work of Kaur et al., 2020,8 the FS was used to identify microbiological features in oral and urinary tract infections, analyzing dental plaque and urine samples. The results indicated that the FS is as effective as traditional microscopes in detecting these pathological features, with the quality of the cellphone camera being a crucial factor. The study also highlighted the didactic use of the FS in promoting oral health in children, with significant improvements in oral hygiene observed in the group that used the FS. This work emphasizes the utility of the FS both in clinical diagnostics and as an educational tool in public health.
In 2021 and 2024, our research group published academic papers highlighting the body projection technique. These papers underscored the technique’s contribution to the education of human anatomy9 and physiology.10 This method utilizes software to overlay anatomical images and physiological videos on models or volunteers, thus enhancing active learning strategies
It promotes a more profound understanding of the human body’s structures and systems, broadening access to basic sciences and increasing its adaptability to diverse educational settings. Similarly, our research has shown that the FS technique presents analogous advantages for histology teaching. Its portability and ease of use facilitate the execution of practical histology exercises in settings where traditionally only theoretical instruction is provided. The FS facilitates the observation of histological structures in a practical and accessible way, overcoming the usual barriers between theoretical and practical learning. This innovation has the potential to transform the teaching of histology, making it more dynamic and aligned with professional practices in the health field.
During our research, challenges in handling and applying the FS were identified. Besides the limitations of magnification and loss of sharpness compared to the traditional method, the need for controlled and uniform lighting proved crucial for image quality. Overexposed images, resulting from lighting failures, can hinder the interpretation and analysis of structures.
Careful operation of the FS was essential to minimize distortions and ensure correct observations. The use of the FS often requires the cooperation of two users to coordinate simultaneous movements and adjustments. This requirement for teamwork adds complexity to the use of the paper microscope, which can be challenging in classroom environments. This limitation, along with the complexity of handling, was also observed in the study by Ganesan et al.11
According to the analyses conducted by the two reviewers, there were no significant differences in the identification of microscopic structures with the FS and optical microscopes using 10x objectives. However, a key limitation of the FS is its fixed magnification capability. In contrast, optical microscopes offer a variety of achromatic objectives with different magnification levels, making them more adaptable and effective for practical histology teaching. This limitation of the FS reduces its applicability for some of the tissues evaluated, allowing only the visualization of limited characteristics. For example, in muscular and testicular tissues, the FS could not clearly display detailed structures such as striations in muscle fibers or the intricate arrangement of cells within the seminiferous tubules.
The fixed magnification limitation of the FS, identified in our study, is consistent with limitations observed in clinical research. Studies by Ephraim et al.1 and Gupta et al.2 which evaluated the diagnostic accuracy of the FS compared to traditional microscopy methods, indicated that the use of the FS might result in low diagnostic sensitivity, highlighting the device’s constraints in applications requiring greater precision. However, the manufacturer of this tool announced that, in 2024, a new version of the FS could be equipped with lenses capable of achieving magnifications of up to 340 times. This will significantly expand the applications of the FS in the educational context, opening new perspectives for future research.
It is important to note that the use of the FS can be based on two aspects: cost and the quality of viewing microscopic structures. Considering that efficacy refers to the capacity to achieve a certain result, and efficiency is related to how an activity is performed, considering aspects such as competence, economy, and productivity, we emphasize the premise that in terms of cost, the efficiency of the FS is superior compared to the optical microscope. However, in qualitative terms, that is, in terms of efficacy, the optical microscope excels. We emphasize that this study was directed toward the efficacy of the FS in teaching basic histology (undergraduate and technical education in the health field). However, the tool cannot be applied for teaching advanced histology.
Despite its limitations in terms of applicability and manipulation, the FS represents a useful tool for bridging the gap between practical and theoretical classes in histology teaching, offering a more integrated and interactive approach. Unlike traditional laboratory settings, the FS allows the study of biological materials in a variety of environments. Depending on the type of sample being analyzed, there is no need for a specific laboratory, enabling students to extend their practical learning experiences beyond the institutional setting—even into their homes.
Conclusion
Version 1.0 (original) of the FS is partially effective in identifying structures that can be visualized with a magnification of approximately 100 times, similar to what is achieved with a 10x objective in optical microscopes. While the FS does not completely replace the optical microscope, it serves as a complementary tool, comparable to virtual microscopy, enriching histology education. By providing a hands-on method for studying histological structures, the FS enriches the educational landscape, expanding the range of learning opportunities for students in histology education.
References
1. Ephraim RK, Duah E, Cybulski JS, Prakash M, D’Ambrosio MV, Fletcher DA, Keiser J, Andrews JR, Bogoch II. Diagnosis of Schistosoma haematobium infection with a mobile phone-mounted Foldscope and a reversed-lens CellScope in Ghana. The American Journal of Tropical Medicine and Hygiene, 2015;92(6):1253-1256. https://doi.org/10.4269/ajtmh.14-0741
Crossref PubMed PMC
2. Gupta S, Mathews BJ, Ghantaa SN, Amerneni KC, Karuna T, Pakhare A, Joshi D, Khadanga S. Foldscope: Diagnostic Accuracy and Feasibility of its Use in National Malaria Control Program. Journal of Microscopy and Ultrastructure, 2021;10(3):114-117. https://doi.org/10.4103/jmau.jmau_103_20
PubMed PMC
3. Parmar DP, Rathod JS, Karkhanawala MM, Bhole PK, Rathod DS. Foldscope: A smartphone based diagnostic tool for fungal keratitis. Indian Journal of Ophthalmology, 2021;69(10):2836-2840. https://doi.org/10.4103/ijo.IJO_3331_20
Crossref PubMed PMC
4. Naqvi A, Manglik N, Dudrey E, Perry C, Mulla ZD, Cervantes JL. Evaluating the performance of a low-cost mobile phone attachable microscope in cervical cytology. BMC Women's Health, 2020;20(1):60. https://doi.org/10.1186/s12905-020-00902-0
Crossref
5. Rameshbabu R, Priya AH, Muthukumar RS, Sivaraman K, Uthra D. Evaluation of Efficacy of Foldscope - A Paper Microscope to be Used as a Chairside Diagnostic Tool in Oral Dysplastic Lesions: A Comparative Study. Contemporary Clinical Dentistry, 2021;12(4):352-358. https://doi.org/10.4103/ccd.ccd_115_20
Crossref
6. Sharma S, Banerjee T, Yadav G, Chandra Chaurasia R. Role of early foldscopy (microscopy) of endotracheal tube aspirates in deciding restricted empirical therapy in ventilated patients. Indian Journal of Medical Microbiology, 2022;40(1):96-100. https://doi.org/10.1016/j.ijmmb.2021.08.004
Crossref
7. Hernández-Pérez C, Nieto-Sobrino M. Foldscope as an Innovative Teaching Tool. Education Sciences, 2022; 12(12):927. https://doi.org/10.3390/educsci12120927
Crossref
8. Kaur T, Dahiya S, Satija SH, Nawal SJ, Kshetrimayum N, Ningthoujam J, Chahal AK, Rao A. Foldscope as a primary diagnostic tool for oral and urinary tract infections and its effectiveness in oral health education. Journal of Microscopy, 2020;279(1):39-51. https://doi.org/10.1111/jmi.12896
Crossref PubMed
9. Hamasaki MY, Mendes C, Neto JP. Body projection: An accessible tool for human anatomy teaching. Education for Health (Abingdon, England), 2021;34(1):37-38. https://doi.org/10.4103/efh.EfH_52_20
Crossref PubMed
10. Hamasaki MY, Mendes C. Animated body projection: a new approach to teach cardiovascular physiology. Advances in Physiology Education, 2024;48(1):102. https://doi.org/10.1152/advan.00266.2023
Crossref PubMed
11. Ganesan M, Selvan Christyraj JRS, Venkatachalam S, Yesudhason BV, Chelladurai KS, Mohan M, Kalimuthu K, Narkhede YB, Christyraj JDS. Foldscope microscope, an inexpensive alternative tool to conventional microscopy-Applications in research and education: A review. Microscopy Research and Technique, 2022;85(11):3484-3494. https://doi.org/10.1002/jemt.24205
Crossref PubMed
© Education for Health.
Education for Health | Volume 37, No. 2, April-June 2024
(Return to Top)


